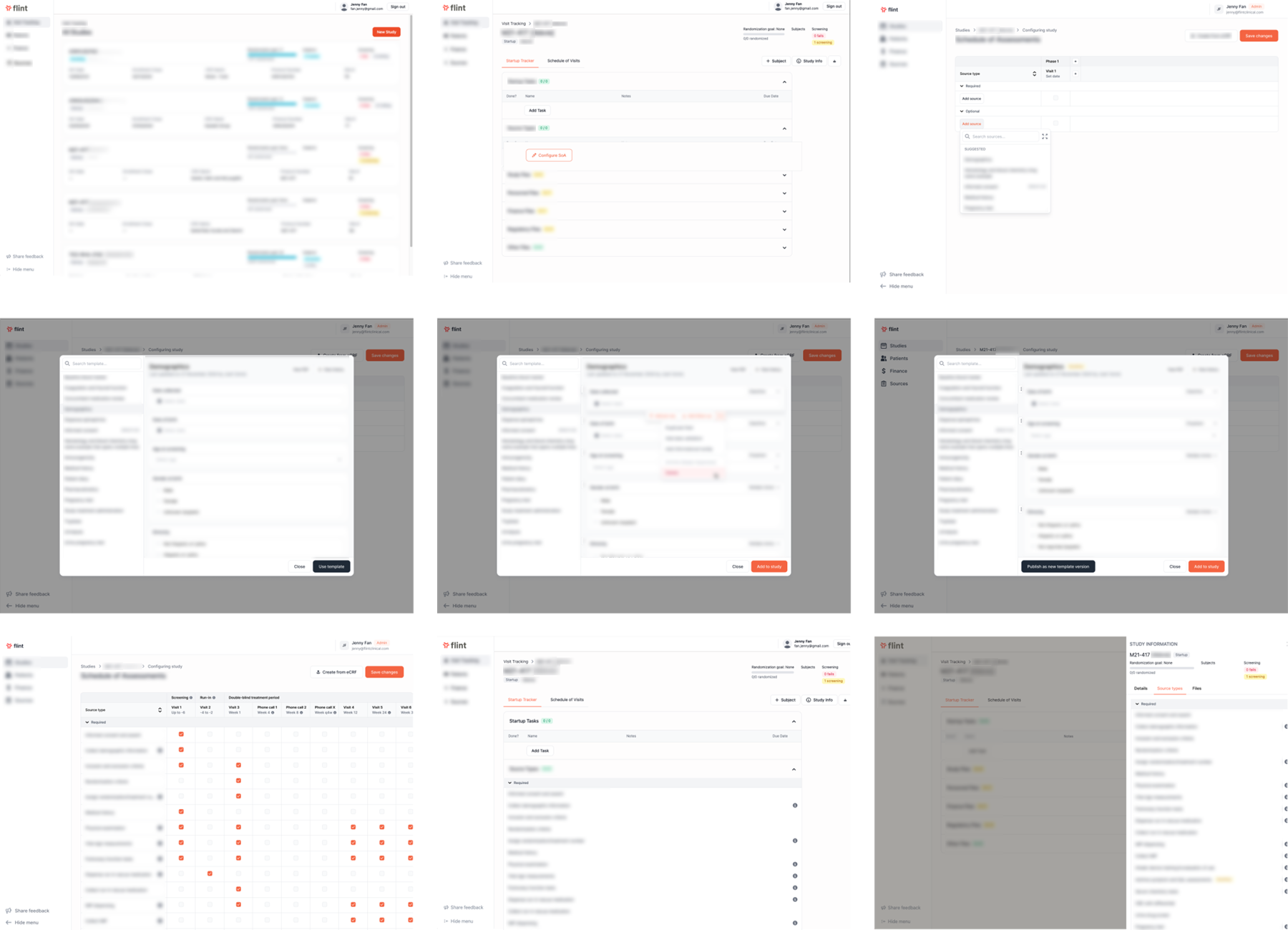
Context
Flint (closed early 2025) was a start-up making cutting-edge clinical trials management software to simplify the study management process, from enrollment through to billing and finance tools. In an industry where studies are often painstakingly managed in Excel spreadsheets and paper documents, Flint aimed to modernize and simplify the process.
Impact
I worked with the founder on a round of sprints to use AI to improve the speed and ease of study source management. Important source research documents have to be maintained for multiple years upon study completion to comply with FDA standards. Normally kept in many paper binders, eSource offered a digital version of tracking the paper trail associated with patient studies, along with added features to improve the ease of creating and maintaining these documents. Through user research, customer outreach/MVP fit discussions, and prototyping new workflows, I shipped designs for eSource that was well-received by early design partners.